#Manufacturing of APIs and Intermediates
Explore tagged Tumblr posts
Text
API Manufacturing | Commercial Manufacturing of APIs and Intermediates
Discover Asymchem's expertise in API manufacturing and commercial production of APIs and intermediates. Learn about our advanced manufacturing processes and commitment to quality.
0 notes
Text

Vidgastech – Ruxolitinib Intermediates Manufacturer
Vidgastech is a leading manufacturer and global supplier of Ruxolitinib Intermediates. We specialize in high-purity pharmaceutical intermediates for API production, supporting the global pharma industry with quality, consistency, and innovation.
#Ruxolitinib Intermediates#Pharmaceutical Intermediates#Ruxolitinib Raw Material#API Supplier#Vidgastech#GMP Manufacturer#Bulk Drug Intermediates#Custom Synthesis
0 notes
Text
Bio-Synth: Redefining Pharmaceutical Excellence in Antihistamines
In today’s competitive pharmaceutical landscape, innovation and quality assurance have become non-negotiable. Bio-Synth, a prominent name in the life sciences sector, stands at the forefront of delivering high-quality antihistamine solutions. With a strong focus on research, compliance, and customer satisfaction, the company is gaining global recognition for its role in advancing healthcare outcomes—particularly in the allergy treatment domain.
Among Bio-Synth's core specialties is the development and supply of advanced molecules for second-generation antihistamines. These include active substances tailored to meet stringent Bilastine API Specification standards. By prioritizing consistency, purity, and bioavailability, Bio-Synth ensures that its products align with international regulatory benchmarks. Their well-characterized antihistamine compounds offer therapeutic reliability, reinforcing the company's commitment to global health.
Another critical area of focus lies in the preparation of essential chemical compounds used in antihistamine production. Bio-Synth has built a strong infrastructure for producing bilastine intermediates, with modern facilities equipped to handle high-volume and high-purity synthesis. This makes the company a trusted choice among partners looking for scalable and reliable sourcing options.
India has emerged as a pharmaceutical manufacturing hub, and Bio-Synth has harnessed this advantage to establish itself among the leading bilastine intermediates manufacturers in India. Leveraging cutting-edge technology and a highly skilled workforce, the organization ensures cost-effective solutions without compromising on quality or compliance.
To conclude, Bio-Synth exemplifies what it means to blend scientific expertise with industrial excellence. With a steadfast commitment to innovation and quality, the company continues to play a pivotal role in the antihistamine sector—delivering value across global markets and setting new benchmarks in pharmaceutical manufacturing.
0 notes
Text

In line with our expanding presence, we continue to collaborate with trusted clients and partners who share our long-term vision. Our consistent focus on precision, quality, and performance shapes every aspect of our service. We cater to sectors like Pharmaceuticals, Agrochemicals, and Specialty Chemicals, backed by robust CRO and CDMO support.
#bioscience#OctaneX Labs#API clinical trial management system#intermediates manufacturers#chemicals API#fine chemical#synthesis#CDMO Companies#CDMO India#life science chemicals#pharmaceutical fine chemicals#capsules#chemicals#cro#cdmo#cdmo companies in india#cdmo services#science#chemical synthesis#chemistry#healthcare#cro services#cdmo lab#cdmo telangana company#custom development projects#custom synthesis#custom development
0 notes
Text
Active Pharmaceutical Ingredient Manufacturers: Powering the Heart of Global Healthcare
In the world of medicine, Active Pharmaceutical Ingredients (APIs) are essential for creating modern drugs. These key components are what make any medication effective and safe. At Chemox Pharma, we're proud to be a reliable name in API manufacturing, providing high-quality products that support healthcare systems around the globe.

What Are Active Pharmaceutical Ingredients (APIs)?
APIs are the main ingredients that give a drug its intended effects. Whether it's for pain relief, fighting infections, or lowering cholesterol, APIs are the ingredients that do the work. High-quality APIs ensure that medicines meet strict global standards and provide consistent results.
Chemox Pharma: A Reliable API Manufacturer
With years of experience and a focus on innovation, Chemox Pharma has become a trusted API supplier in India. Our advanced research and development, GMP-certified facilities, and strict quality control set us apart. We have a broad range of APIs, including:
Atorvastatin Calcium
Citicoline Sodium
Emtricitabine
Fenofibrate
Fexofenadine Hydrochloride
Fluconazole
Nitrofurantoin
Sildenafil Citrate
Tadalafil Citrate
Tigecycline
Dorzolamide
Mirabegron
Sacubitril & Sacubitril Valsartan Sodium
Each product is created with precision to comply with regulations in markets like the US, Europe, Asia, and Latin America.
Focus on Quality and Compliance
At Chemox Pharma, we adhere to the best practices in manufacturing and testing. Our facilities are regularly inspected to meet WHO-GMP and other standards. We strictly follow ICH guidelines and provide full traceability for all our APIs, ensuring compliance in global markets.
Innovation and Global Reach
What makes Chemox Pharma stand out is our commitment to ongoing innovation. We invest in the latest technology and skilled personnel to keep up with market trends and changing therapeutic needs. Our global presence is growing rapidly, with long-lasting partnerships across several continents. We collaborate closely with pharmaceutical companies, research institutions, and healthcare providers to create reliable, cost-effective solutions.
Partnering for a Healthier Future
The demand for dependable API manufacturers in India is on the rise, driven by the need for quality, affordability, and scalability. Chemox Pharma is dedicated to meeting these needs through continuous innovation, responsible manufacturing, and customer-focused service.
Looking for reliable API solutions? Team up with Chemox Pharma – your trusted partner for pharmaceutical API manufacturing that supports a healthier world. For more information you check our site: https://chemoxpharma.com/
0 notes
Text

Monday CRM offers customizable and automated workflows that reduce manual tasks and improve sales tracking. Its flexible boards and automation rules help teams align sales activities with strategic goals and adapt quickly to market changes.
#bioscience#OctaneX Labs#API clinical trial management system#intermediates manufacturers#chemicals API#fine chemical#synthesis#CDMO Companies#CDMO India#life science chemicals#pharmaceutical fine chemicals#capsules#it#technology#it jobs#tech#crm benefits#crm services#sierra consulting#current events#technews#crm#crm strategy#sales crm#crm platform#crm integration#crm software#crm solutions#businesssolutions#business growth
0 notes
Text
API Intermediates Manufacturers in India
Chempro is a trusted manufacturer and supplier of high-quality API intermediates in India. A list of API (Active Pharmaceutical Ingredients) Intermediates Manufacturers in Mumbai, India, including intermediate names, CAS numbers, and corresponding API names.
Visit Us: https://www.chemprogroup.com/pharma/api-intermediate.html
0 notes
Text
#Kekulepharma#Api manufacturer#Api supplier#intermediate#Intermediates supplier#Intermediate manufacturer#Pharmacy#Pharma#Pharma in Hyderabad#pharmabusiness#pharmaindustry
0 notes
Text
In the Indian capital, Book Drug makes a large number widely appealing things for exporters. We produce magnificent midway things at sensible expenses
1 note
·
View note
Text
Pharmaceutical Intermediates Manufacturers | Saurav Chemicals
Explore Saurav Chemicals for top-notch pharmaceutical intermediates, ensuring reliability, a broad spectrum of options, and adherence to GMP standards to enhance the success of your pharmaceutical synthesis endeavors. Rely on our trusted quality for optimal results in your pharmaceutical projects.
#Pharma Intermediates#API Intermediates Manufacturers#Pharma Intermediates manufacturers#Pharmaceutical Intermediates manufacturers
1 note
·
View note
Text
Your Trusted Ticagrelor API Manufacturer for Quality and Reliability
When it comes to Ticagrelor Active Pharmaceutical Ingredient (API) manufacturing, quality and reliability are paramount. As a trusted name in the pharmaceutical industry, Riverx Lab takes pride in being a premier Ticagrelor API manufacturer. With our unwavering commitment to excellence, cutting-edge facilities, and a focus on regulatory compliance, we strive to meet the diverse needs of pharmaceutical companies worldwide. Discover why Riverx Lab is the partner of choice for Ticagrelor API manufacturing.

Section 1: Uncompromising Quality Assurance
At Riverx Lab, quality is the foundation of our manufacturing process. We adhere to stringent quality control measures at every stage, from sourcing raw materials to final product release. Our state-of-the-art facilities are equipped with advanced technologies and operated by a skilled team of experts who follow current Good Manufacturing Practices (cGMP) guidelines. By maintaining the highest quality standards, we ensure that our Ticagrelor API consistently meets the stringent requirements of pharmaceutical manufacturers and regulatory authorities.
Section 2: Cutting-Edge Manufacturing Facilities
Riverx Lab boasts cutting-edge manufacturing facilities designed to optimize efficiency and productivity while maintaining the highest level of product integrity. Our facilities adhere to international standards and incorporate advanced equipment for synthesis, purification, and analysis. By leveraging modern technologies and automation, we streamline the manufacturing process, minimize variation, and deliver Ticagrelor API with exceptional purity and consistency.
Section 3: Expertise and Experience
As a Ticagrelor API manufacturer, Riverx Lab possesses a team of seasoned professionals with extensive experience in pharmaceutical manufacturing. Our researchers, chemists, and engineers work collaboratively to develop and refine innovative processes, ensuring the highest quality and efficiency in Ticagrelor API production. With their in-depth knowledge and expertise, we consistently deliver products that meet or exceed customer expectations.
Section 4: Regulatory Compliance
Pharmaceutical manufacturing demands strict adherence to regulatory guidelines and standards. Riverx Lab recognizes the importance of compliance and operates in full compliance with international regulatory requirements. Our facilities undergo regular inspections and audits to ensure adherence to current regulations, including the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), the United States Food and Drug Administration (FDA), and the European Medicines Agency (EMA). By choosing Riverx Lab as your Ticagrelor API manufacturer, you can be confident in receiving products that meet the highest regulatory standards.
Section 5: Customized Solutions and Timely Delivery
At Riverx Lab, we understand that every pharmaceutical manufacturer has unique requirements. We offer flexible solutions and customization options to meet your specific needs. Our team works closely with you to ensure that the Ticagrelor API we provide aligns with your project timelines, quantity requirements, and quality specifications. With our efficient supply chain and commitment to on-time delivery, you can rely on Riverx Lab to meet your production demands and keep your projects on schedule.
Conclusion:
Riverx Lab stands as a trusted Ticagrelor API manufacturer, driven by a passion for excellence, innovation, and regulatory compliance. With our unwavering commitment to quality assurance, cutting-edge facilities, and experienced team, we deliver Ticagrelor API products that meet the highest industry standards. Partner with Riverx Lab to experience our dedication to reliability, customization, and timely delivery. Contact us today to discuss your Ticagrelor API manufacturing needs and embark on a successful partnership with a leading industry expert.
0 notes
Text
Exploring the Global Aldehydes Market: Key Players and Market Dynamics
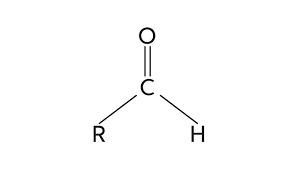
The aldehydes market is a segment of the chemical industry that deals with the production and distribution of a class of organic compounds known as aldehydes. These compounds are characterized by the presence of a carbonyl group (C=O) bonded to a hydrogen atom and a carbon atom in their chemical structure. Aldehydes find widespread applications in various industries, thanks to their unique properties and versatile reactivity.
In terms of market overview, the aldehydes market has been experiencing steady growth in recent years. This growth can be attributed to the increasing demand for aldehydes in industries such as pharmaceuticals, agriculture, food and beverages, and cosmetics. Aldehydes serve as crucial intermediates in the synthesis of various chemicals and are essential in the production of fragrances, flavor enhancers, and pharmaceuticals.
The growth in the aldehydes market industry can be primarily attributed to the expansion of these end-user industries. For instance, the pharmaceutical industry relies heavily on aldehydes for the synthesis of a wide range of drugs and active pharmaceutical ingredients (APIs). Additionally, the food and beverage industry utilizes aldehydes for flavor enhancement and preservation purposes, further driving market growth.
The aldehydes market is also influenced by evolving industry trends. One significant trend is the increasing emphasis on green chemistry and sustainable practices. Many companies in the aldehydes sector are adopting environmentally friendly production processes, such as catalytic hydrogenation, to reduce the environmental impact of their operations. This trend aligns with the growing awareness of environmental issues and the need for more eco-friendly chemical manufacturing.
Another noteworthy trend is the constant innovation and development of novel aldehyde derivatives with enhanced properties. This innovation is driven by the demand for higher-quality products in various industries. Researchers and manufacturers are continuously exploring new applications and synthesizing aldehydes tailored to meet specific industry requirements, which contributes to market expansion.
In conclusion, the aldehydes market is a dynamic segment within the chemical industry, driven by the increasing demand from various end-user industries. As industries continue to grow and evolve, the market is expected to witness further advancements, particularly in sustainable production methods and novel aldehyde derivatives, to meet the changing needs of consumers and businesses alike.
2 notes
·
View notes
Text
Ruxolitinib Intermediates: Enabling Targeted Therapy with Quality Manufacturing from Vidgastech
In the rapidly evolving world of pharmaceutical research and precision medicine, Ruxolitinib has gained significant attention as a JAK1/JAK2 inhibitor used to treat conditions like myelofibrosis, polycythemia vera, and other rare blood cancers. To ensure effective and scalable drug development, the demand for high-quality Ruxolitinib intermediates has surged — and that’s where Vidgastech leads the way.
🔬 What Are Ruxolitinib Intermediates?
Ruxolitinib intermediates are the essential chemical compounds used during the synthesis of the final active pharmaceutical ingredient (API), Ruxolitinib. These intermediates must be manufactured with strict quality standards to ensure:
Purity and consistency
Scalability for formulation
Compliance with global pharmacopeia
🏭 Vidgastech: A Trusted Source for Pharma Intermediates
Vidgastech has established itself as a reputable manufacturer and exporter of pharmaceutical intermediates in India, specializing in cutting-edge molecules like Ruxolitinib intermediates. With modern production facilities, a skilled R&D team, and commitment to regulatory standards, Vidgastech ensures:
GMP-compliant manufacturing
Customized synthesis on request
Prompt global delivery
💡 Why Choose Vidgastech for Ruxolitinib Intermediates?
High Purity Compounds: Ensuring effectiveness in the final API.
Regulatory Compliance: Following stringent quality checks.
Custom Solutions: Tailored synthesis as per client specifications.
Reliable Supply Chain: On-time delivery across the globe.
🌍 Applications in Oncology
Ruxolitinib is a vital component in the treatment of:
Myelofibrosis
Polycythemia Vera
Graft-Versus-Host Disease (GVHD)
Ongoing trials for autoimmune conditions
With the growth of targeted therapies, Ruxolitinib intermediates play a foundational role in pharmaceutical innovation.
🔗 Connect with Vidgastech
If you're a pharmaceutical manufacturer, researcher, or procurement manager looking for trusted sources of Ruxolitinib intermediates, Vidgastech offers scalable solutions tailored to your needs. 🌐 Website: https://www.vidgastech.com
#Ruxolitinib Intermediates#Pharmaceutical Intermediates#API Manufacturing#JAK Inhibitor Intermediates#Ruxolitinib Suppliers India#Vidgastech#Oncology Intermediates#Drug Synthesis#Pharma Chemicals#GMP Manufacturing#Custom Synthesis#Myelofibrosis Treatment#Pharmaceutical Exporters#Life Science Chemicals#Pharma R&D India#High Purity Intermediates#Bulk Drug Intermediates#Specialty Chemicals Manufacturer#Healthcare Industry India#Pharmaceutical Industry News
0 notes
Text
Innovative Solutions in the Production of Bilastine Intermediates and APIs
The pharmaceutical industry constantly evolves, with companies developing more advanced and efficient ways to produce key drugs. Bilastine, a popular antihistamine used to treat allergies, has seen a rise in demand in recent years. The production of its active pharmaceutical ingredient (API) and intermediates plays a crucial role in meeting this demand. Bilastine intermediates manufacturers in India have emerged as a key part of the global supply chain, contributing to the production of high-quality Bilastine products.
The Bilastine API specification sets strict guidelines that manufacturers must follow to ensure consistency, safety, and efficacy in the final product. These specifications detail the necessary quality standards for the Bilastine API, including purity, identification, and performance characteristics. Adhering to these specifications is essential for maintaining the integrity of the drug and its therapeutic effects.
India, with its robust pharmaceutical manufacturing industry, has become a leading player in the production of Bilastine intermediates. Many manufacturers in India specialize in the production of chemical intermediates, which are essential for the synthesis of the active ingredient. These intermediates are used in the final stages of Bilastine synthesis, making their quality critical to the success of the drug.
Conclusion
The increasing demand for Bilastine has spurred innovations in the production of its intermediates and APIs. As global pharmaceutical companies look to expand their supply chains, the role of Bio-synth and other industry leaders in providing high-quality intermediates and APIs cannot be overstated. With continued advancements in manufacturing processes, the industry is poised to meet the growing global demand for Bilastine and similar pharmaceutical products.
0 notes
Text

As we evolve, we align with valued partners and clients who believe in our direction and actively support our momentum. Quality assurance and operational excellence are deeply ingrained in our work ethic. Our portfolio includes Pharmaceuticals, Agro-based products, Specialty Chemicals, and a complete range of CRO and CDMO offerings.
#bioscience#OctaneX Labs#API clinical trial management system#intermediates manufacturers#chemicals API#fine chemical#synthesis#CDMO Companies#CDMO India#life science chemicals#pharmaceutical fine chemicals#capsules#chemicals#cro#cdmo#cdmo companies in india#cdmo services#science#chemical synthesis#chemistry#healthcare#cro services#cdmo lab#cdmo telangana company#custom development projects#custom synthesis#custom development
0 notes
Text
API Manufacturing Through CDMOs: A Strategic Approach to Supply Chain Resilience

In an increasingly interconnected and volatile world, pharmaceutical supply chains face numerous disruptions—from geopolitical tensions and raw material shortages to regulatory changes and pandemics. At the heart of pharmaceutical manufacturing lies the Active Pharmaceutical Ingredient (API)—the critical component responsible for the therapeutic effect of a drug. Ensuring a consistent, high-quality supply of APIs is essential for drug availability and patient health. To achieve this, pharmaceutical companies are turning to Contract Development and Manufacturing Organizations (CDMOs) as a strategic solution to bolster supply chain resilience.
The Evolving Role of CDMOs in API Manufacturing
Traditionally, CDMOs were seen primarily as outsourcing partners for overflow production or cost-cutting. However, as pharmaceutical companies face greater complexity and unpredictability in their global operations, CDMOs have evolved into strategic partners that offer not just manufacturing capabilities, but also technical expertise, regulatory compliance, and supply chain flexibility.
In the context of API manufacturing, CDMOs provide:
End-to-end services, including process development, scale-up, and commercial production.
Access to advanced technologies such as continuous manufacturing, high-potency handling, and green chemistry.
Expertise in handling complex APIs including peptides, cytotoxics, and controlled substances.
Why Supply Chain Resilience Matters in API Production
APIs often rely on raw materials or intermediates sourced from a few countries, which can make pharmaceutical companies vulnerable to disruptions. For instance, the COVID-19 pandemic exposed global dependence on select regions for critical drug ingredients. Even a temporary shutdown or port delay in one country can impact the global supply of life-saving medications.
In this scenario, CDMOs help mitigate risk by offering:
Geographically diversified manufacturing sites to reduce regional dependency.
Redundant production capacity that ensures continuity in case of local disruptions.
Vendor management and second-source qualification, providing security against raw material shortages.
By partnering with CDMOs, companies can implement agile manufacturing strategies that respond quickly to fluctuations in demand or supply disruptions.
CDMO Pharmaceuticals: Enhancing API Quality and Compliance
Manufacturing APIs is not just about yield and efficiency—it requires strict adherence to Good Manufacturing Practices (GMP), regulatory guidelines, and environmental standards. CDMO pharmaceuticals bring extensive experience navigating global regulatory frameworks such as those of the FDA, EMA, PMDA, and WHO.
Many CDMOs invest heavily in:
Quality systems and digital batch records for traceability.
Analytical method development and validation to ensure product purity and potency.
Regulatory intelligence teams that assist clients in dossier preparation and submission.
This level of quality control is crucial for companies looking to enter global markets or file ANDA, NDA, or MA submissions with confidence.
Flexibility and Speed-to-Market
One of the most pressing challenges for pharmaceutical companies, especially in generics and specialty drugs, is the need for speed-to-market. Developing and validating API manufacturing processes in-house can be time-consuming and expensive.
CDMOs offer a ready-made infrastructure, skilled workforce, and technology platforms that allow faster:
Process development and optimization
Technology transfer
Scale-up from clinical to commercial production
This agility is particularly valuable in the context of drug shortages, pandemic response, or expedited development pathways such as Orphan Drug or Fast Track designations.
Building a Future-Ready Supply Chain
The pharmaceutical landscape is shifting toward resilience over cost alone. Partnering with the right CDMO creates a collaborative ecosystem that enhances not only operational efficiency but also long-term competitiveness.
By outsourcing API manufacturing to CDMOs, companies can:
Reduce capital expenditure.
Focus internal resources on R&D and commercialization.
Gain peace of mind knowing their supply chain is robust, scalable, and compliant.
In this regard, cdmo pharmaceuticals are becoming indispensable players—not just service providers, but strategic partners in delivering global health.
Conclusion
In today’s uncertain environment, API manufacturing through CDMOs represents more than operational outsourcing—it’s a strategic investment in supply chain resilience. With their technical expertise, regulatory knowledge, and global reach, CDMOs are enabling pharmaceutical companies to navigate complexities, safeguard production, and deliver essential medicines without interruption. As the demand for secure, compliant, and scalable API production grows, the role of CDMO pharmaceuticals will only become more critical in shaping the future of global healthcare.
0 notes